The objective of this paper is to depict the distinctive kind of gels that are probably going to happen in polyolefin film items, procedures for distinguishing the gel compose, and specialized answers for moderate them from single-screw expulsion forms.
Conventions for Gel Analysis in Polymers
Set up conventions for gel investigation in polymer films are all around reported in the writing. For instance, gels can be recognized utilizing the schematic procedure appeared in Figure 1. Ordinarily, a film with deformities is outwardly investigated utilizing a low power dismembering magnifying instrument. The gels can be grouped in view of size, shading, and shape, and disconnected utilizing an extremely sharp edge or scissors. Cross areas of the gels going from 5 µm to 10 µm thick are gathered at temperatures beneath the glass change temperature (Tg) of the film utilizing a cryogenic microtome; i.e., – 80°C to – 120°C. For optical examination, a thin segment containing the gels is set on a glass magnifying lens slide with a drop of silicon oil and secured with a glass coverslip. Extra segments are gathered for examination by means of hot stage microscopy and for compositional recognizable proof if necessary.
Subsequent to gathering the segments, the cleaned square face containing the rest of the gel is held. In numerous cases, gels emerge from inorganic contaminants, for example, the metallurgy from pellet taking care of hardware, extruders, or segments from masterbatches. Examination of these inorganic parts is best performed with the blackface test utilizing a checking electron magnifying lens (SEM) furnished with a vitality dispersive x-beam identifier (EDX) [5,6]. Sometimes, added substances or inorganic buildups are available in low fixations inside the gels. A technique to advance the grouping of these materials is to uncover the square face containing the gel to oxygen plasma. Scratching will specially expel the polymer at a considerably quicker rate than the inorganic materials, improving the inorganic segments for natural investigations. It must be noticed that before SEM and EDX investigations, a thin conductive covering like carbon is ordinarily vanished onto the example to render it conductive under the electron pillar
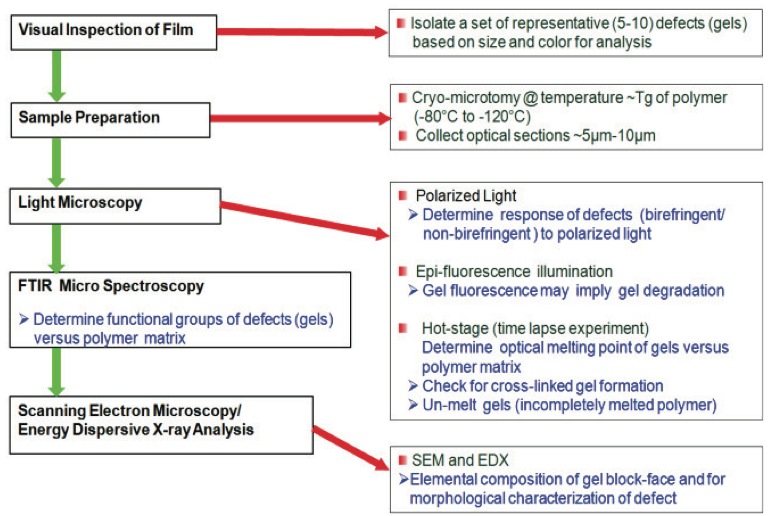
Figure 1. Technique for describing surrenders in polymer films
The following areas will exhibit different techniques for examination utilized for basic gel composes.
Oxidized Gels in Polymers
The most widely recognized sort of gel is caused by oxidative procedures that crosslink the PE chains. The most ideal approach to distinguish this gel compose is by watching them with spellbound light and bright (UV) light sources. Transmitted energized light microscopy speaks to a powerful procedure [7] that can be utilized to examine structures in crystalline movies. For instance, dark spot gels were debasing a multilayer film item. The gels were moderately fragile when cutting for examination. The source was obscure. The detail of a gel is plainly obvious utilizing transmitted enraptured light, as appeared in Figure 2a. Close examination of this gel utilizing epi-fluorescence with a bright light source caused a serious fluorescence outflow, as appeared in Figure 2b. This kind of discharge recommends warm oxidation and crosslinking of the polymer. Small-scale infrared investigation of the gel demonstrated that it contained oxidized PE and maleic anhydride, as appeared by the range in Figure 3 (for clearness, this figure can be found toward the finish of this paper). This material likely shaped on the metal surfaces of the extruder and after that chipped off amid a minor procedure unsteadiness. The material at that point streamed downstream and sullied the film as a gel.

Figure 2. Transmitted energized light pictures of a thermally oxidized and crosslinked gel in a multilayer polymers film: a) photo in enraptured light, and b) the gel fluorescing under UV light.
Crosslinked Gels in Polymers
Crosslinked gels are oxidized gels, yet the level of oxidation may not be sufficient to make them fluoresce under UV light. These gels may have a level of crystallinity and along these lines be birefringent under the captivated light. For instance, the marginally birefringent gel appeared in Figure 4a was contemplated utilizing a temperature programmable hot stage, polarizing light magnifying lens [7]. The optical softening temperature (Tm) of the gel was estimated at 128°C and predictable with the PE used to make the item, as appeared in Figure 4b. To decide whether the gel was unmixed (exceptionally entrapped yet not crosslinked), the gel was held over the softening temperature (135°C) and afterward focused. A dental device was utilized to pressure the highest point of the glass coverslip. Crosslinked gels will seem birefringent, (Figure 4c) because of the anisotropy of stress conveyance in the gel to spellbound light. The gel measurements and shape stayed in the wake of cooling confirming crosslinking, as appeared in Figure 4d. In the event that the gel was profoundly caught and not crosslinked, the gel would have vanished after the pressure and cooling were connected.
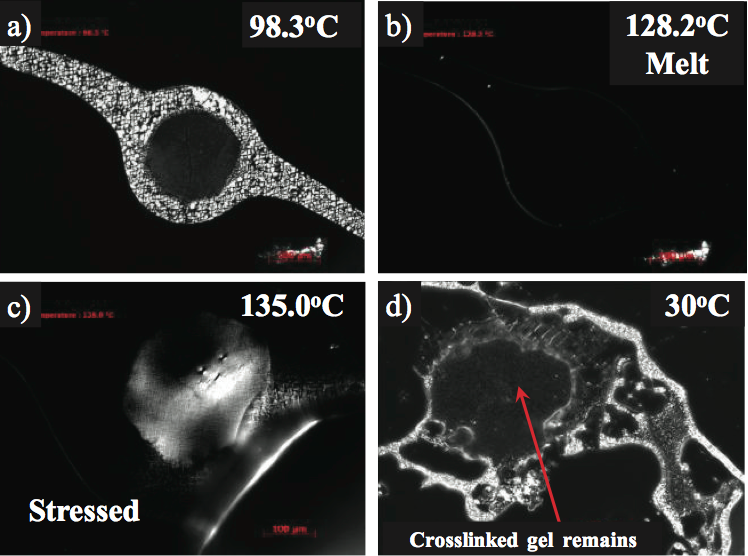
Figure 4. Hot stage microscopy of a crosslinked gel in a crystalline monolayer polymers film: a) beneath the liquefying temperature, b) optical softening point at 128°C, c) appearance of birefringence in the wake of worrying at 135°C, and d) unblemished crosslinked gel in the wake of cooling to 30°C.
Gels from Foreign Contamination
The beginning of imperfections causing staining in polyolefin pellets can be recognized utilizing light and electron microscopy. For instance, PE pellets from an in-plant reuse re-pelletizing process contained pellets that were offensive and had dark spots, as appeared in Figure 5a. One of these deformities was segregated utilizing the cross-segmenting procedure, as appeared in Figure 5b. The cross area uncovered an exceptional rosy molecule that caused the staining of the pellet.
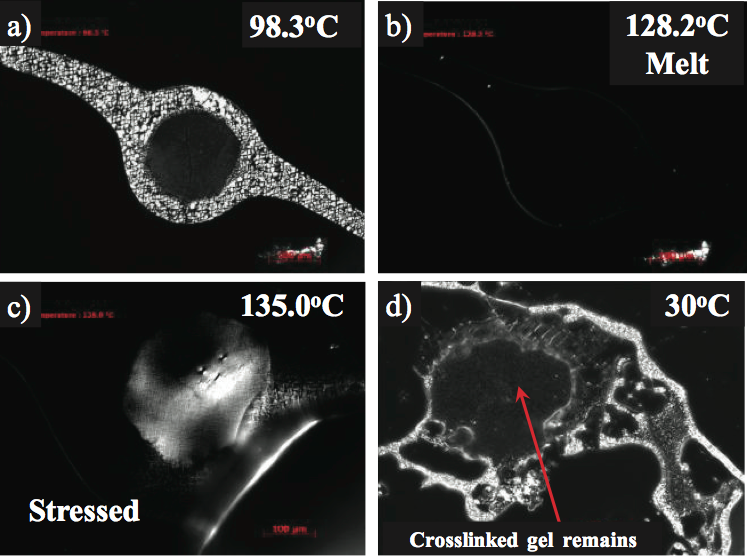
Figure 5. Photos of remote sullying in pellets of a re-pelletized recover stream: a) photomicrograph of stained polyolefin pellets containing dim deformities, and b) transmitted spellbound light micrograph of a pellet cross segment containing an imperfection.
SEM and EDX microanalysis were utilized to confirm that the deformities contained fundamentally iron and oxygen, and it likely was iron oxide. Figure 6 demonstrates a backscatter electron picture (BEI) of the pellet square face test demonstrating the imperfection causing the staining and the basic range. Metallic based deformities can start from process gear, railcars utilized for shipment, pellet exchange lines, and poor housekeeping. The birthplace of the iron oxide was likely from a capacity canister.
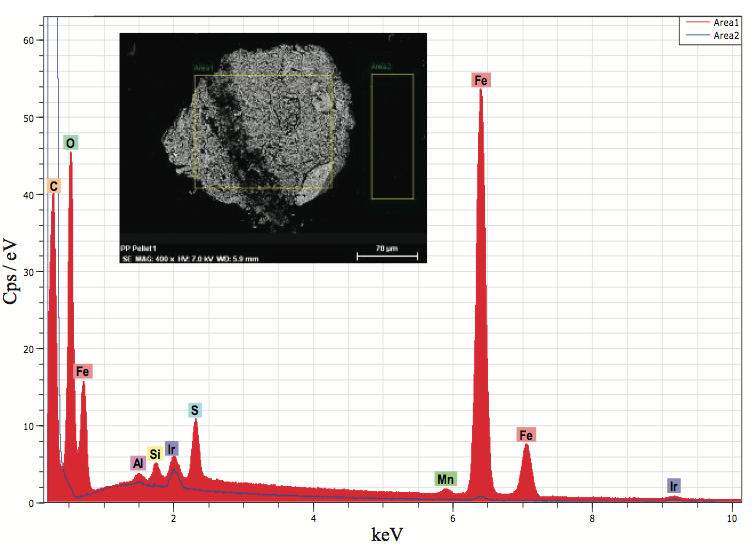
Figure 6. EDX microanalysis of an incorporation in a polymers polyolefin pellet cross segment (Figure 5b). The examination showed that the molecule was likely iron oxide.
In another precedent, a multilayer film item was encountering incidental gels. The gels were disconnected and the cross segments were gathered as appeared in Figure 7a. These gels contained exceedingly birefringent particles that dwelled in the center layer. The external film layers seemed shapeless and the center layer was somewhat birefringent. The optical softening temperature of the center layer was resolved to be 123°C while the birefringent gels dissolved at 265°C. The dissolving temperature of 123°C was predictable with the PE tar used to create the center layer. The higher dissolving temperature material and small-scale infrared investigations of the deformities show that they were outside contaminants, and they were distinguished as a polyester tar. The polyester sap was utilized in another procedure in the changing over the plant, and it incidentally defiled the PE feedstock.
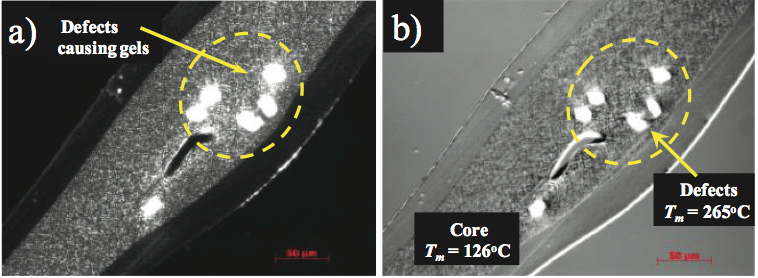
Figure 7. Photos of gels in the center layer of a three-layer polymers film: a) transmitted captivated light, and b) hot stage microscopy was utilized to decide the liquefying temperatures of the center tar and imperfections.
Another regular contaminant that produces gels is stranded, as appeared in Figure 8. As a rule, these contaminants are cotton filaments from dress and gloves or cellulosic strands from bundling materials. Fourier change infrared (FTIR) spectroscopy is extraordinary compared to other methods for deciding the concoction usefulness of naturally based deformities in PE films. The infrared absorbance qualities of the imperfection were resolved to utilize FTIR spectroscopy, as appeared in Figure 9 (for lucidity, this figure can be found toward the finish of this paper). The expansive ingestion groups close to 3600 cm-1 to 3100 cm-1 are because of hydroxyl (- OH) extending vibrations, the C-H vibration extend is almost 2916 cm-1 to 2851 cm-1, and the ester carbonyl gathering retention is almost 1734 cm-1. In view of the infrared retention attributes, the imperfection in the PE film is a cellulosic fiber with debased PE gum.
Once the contaminant is distinguished, the troubleshooter must decide how the material entered the feedstock stream. Process controls must be distinguished and executed to relieve the contaminant source.
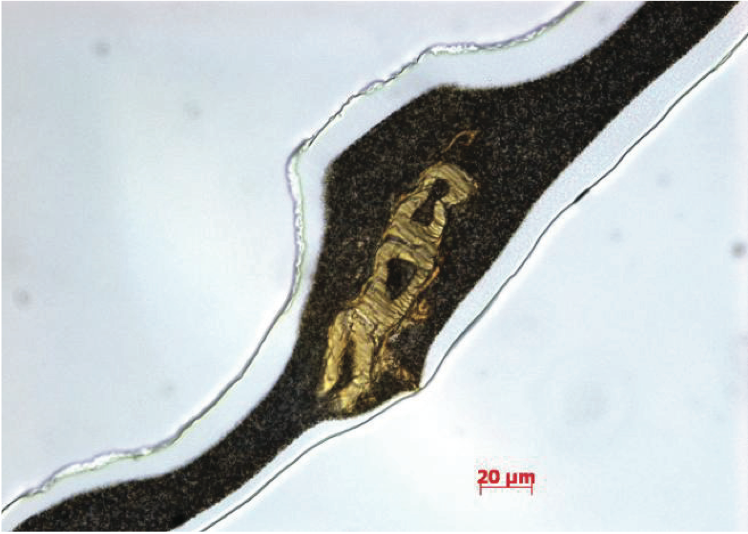
Figure 8. Transmitted brilliant field picture of PE polymers film containing a sinewy gel.


2 Comments
Thanks, it is very informative
Thanks for the terrific article