Human exposure and the toxicity of the substance(s) like the plastic filler used in the formulation of FCMs are the two main components that constitute a risk assessment in relation to human health. This section presents a general overview of the risk and exposure assessment approaches from EFSA and on practices of national bodies as reported by the members of the EFSA network to JRC queries. It also includes information collected from the industrial professional associations.
It should be noted that, in Europe, the risk assessment was conducted by the Scientific Committee for Food (SCF) from 1991 to 2002. Since 2002, Regulation (EC) No 178/2002 (general food law) has established EFSA as the independent EU body that conducts risk assessments related to food safety. The FCM framework legislation also states that provisions liable to affect public health shall be adopted after consulting EFSA. The SCF introduced a tiered approach for risk assessment of plastic FCMs, which is still used by EFSA.
The European Food Safety Authority (EFSA)
EFSA has published guidelines on the authorisation process for substances in materials that are regulated at EU level, i.e.:
- Plastics ( please check the EFSA — Guidance document on the submission of a dossier on a substance to be used in FCMs for evaluation, j.efsa.2008.21r.).
- Active and intelligent substances (please check the EFSA — Guidelines on submission of a dossier for safety evaluation by EFSA of active or intelligent substances present in active and intelligent materials and articles intended to come into contact with food. j.efsa.2009.1208.
EFSA has also published a guidance document for recycling processes (please check the EFSA — Guidelines on submission of a dossier for safety evaluation by the EFSA of a recycling process to produce recycled plastics intended to be used for the manufacture of plastic materials and articles in contact with food — j.efsa.2008.717.) to produce recycled plastic to be used in contact with food, which contained criteria (please check the EFSA — Scientific opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended to be us the d for manufacture of materials and articles in contact with food. EFSA Journal 2011;9(7):2184.) to be used for the safety evaluation of a mechanical recycling process to produce recycled PET.
EFSA has published several documents on the risk assessment of chemicals in general and that may be of relevance to substances present in food as a result of migration from FCMs. These include documents on the use of the concept of threshold of:
- Toxicological concern (check the EFSA — Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree.
- On human risk assessment of combined exposure to multiple chemicals (International frameworks dealing with human risk assessment of combined exposure to multiple chemicals, j.efsa.2013.3313.).
- On the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain (EFSA — Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain, j.efsa.2011.2140).
- On risk assessment terminology (EFSA — Scientific Opinion on Risk Assessment Terminology, j.efsa.2012.2664.).
- On genotoxicity testing strategies applicable to food and feed (EFSA — Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment, j.efsa.2011.2379.).
- On proposed harmonised default values for use in risk assessment (EFSA — Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data, efsa.2012.2579.).
- On the applicability of the margin of exposure approach for impurities which are both genotoxic and carcinogenic (EFSA — Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed, j.efsa.2012.2578.).
EFSA also has a comprehensive European food consumption database (EFSA — Use of EFSA Comprehensive European Food Consumption Database for estimating dietary exposure to genetically modified foods, j.efsa.2015.4034.) that feeds into the exposure part of the risk assessment.
EFSA recently published a report on developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances used in food contact materials (EFSA — Scientific opinion on recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances used in food contact materials, j.efsa.2016.4357.), which also addresses a potential impact on EFSA’s processes.
An EFSA Scientific Cooperation Working Group collected information on non-plastic FCMs to anticipate emergency situations linked to the potential presence in food of substances released from FCMs (ESCO report).
National and professional guidance in the EU for plastic and related substances
The EFSA ESCO report analysed the situation and risk assessment approaches in European countries in its Section III.A, ‘Overview of the risk assessment and of the national Regulations, as provided by the MSs’. JRC desk research used this information along with two rounds of feedback from EFSA’s FIP-network and NRLs. The information from these sources in regard to risk assessment is included in the article Summary of information for risk assessment.
Risk assessment bodies for food contact issues can be identified for Belgium, the Czech Republic, Germany, Greece, Spain, France, Croatia, Italy, Lithuania, Hungary, the Netherlands, Norway, Poland, Finland, and Switzerland, whereas Luxembourg reported not having a risk assessment body. Slovakia does the risk assessment as part of the work of its NRL for FCMs.
Risk assessment procedures specific to food contact are in place for 10 countries (Austria, the Czech Republic, Denmark, France, Germany, Italy, the Netherlands, Poland, Slovenia and Switzerland), and they perform assessments.
Thirteen MSs report that they have a risk assessment procedure but few or no assessments have been done, or that they have a process not specifically designed for FCMs (Croatia, Cyprus, Finland, Greece, Hungary, Ireland, Latvia, Luxembourg, Norway, Slovakia, Spain, Sweden and the United Kingdom). Two MSs (Estonia, Lithuania) report that they are in the set-up phase of a risk assessment procedure for food safety. Figure 1 illustrates the extent of risk assessment in EU MSs.
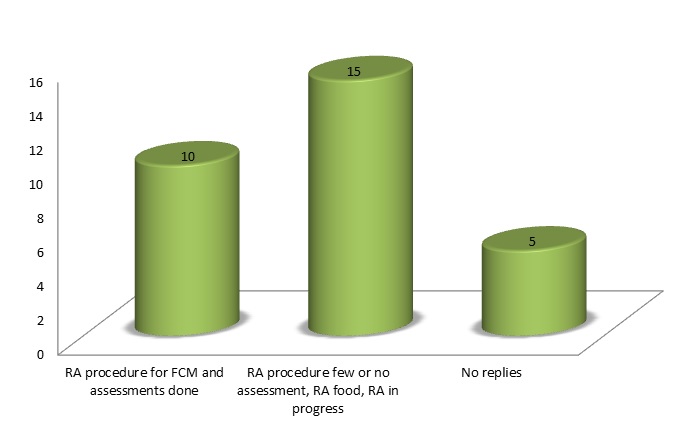
Figure 1: RA extent in EU + Norway and Switzerland
A relevant proportion of MSs (40 %) report using the SCF-FCM guidelines and the EFSA note for guidance for plastic FCMs or a mix of EFSA or other references depending on the type of materials (for the non-harmonised ones). Examples include the Czech Republic, Germany, Ireland, France, Croatia, Italy, Hungary, the Netherlands, Austria, Slovakia, Finland, and Switzerland. Denmark, Cyprus and Slovenia state that they have their own approach.
Two types of risk assessment approaches for FCMs are identified. One approach is to assess the risk of the individual substances used in FCMs, to allow risk management decisions to be made on the relevant restrictions or absence of restrictions (in legislation or recommendations). This goes in conjunction with the assessment of the risks of non-compliance during official controls and possible follow-up RASFF notifications. Countries that follow this approach are the Czech Republic, Denmark, France, Germany, Italy, Norway, Poland, the Netherlands, and Switzerland. The Czech Republic, France, Germany, Italy, the Netherlands, Poland and Switzerland report that they use the SCF-FCM guidelines and/or the EFSA note for guidance for plastic FCMs in the risk assessment of a substance.
The other main risk assessment approach cited is to focus on the assessment of the risks of non-compliances in official controls and RASFF notifications only. MSs using this approach, such as Finland, Ireland, Greece, Croatia, Cyprus, Latvia, Luxembourg, Hungary, Austria, Slovenia and the United Kingdom, may use all types of sources for assessing the risk of non-compliance, including risk assessment information from other MSs. The information given by Belgium and Cyprus does not allow categorisation of their risk assessment approach yet.
Considering national risk assessment procedures with respect to those of EFSA, specific protocols for authorisation of FCM substances developed at national level appear to differ to some extent from the EFSA procedure in the case of non-harmonised FCMs. The main deviations that could be identified in the context of this study are on the exposure assessment (Germany, the Netherlands), migration testing (Italy, the Netherlands, Switzerland) and toxicity data (France, the Netherlands).
Germany has specific guidance for the determination of exposure to (chemical) substances from paper and board. France states in the ESCO report that a substantial proportion of substances on the lists of authorised substances have been evaluated on the basis of a reduced dossier, not in agreement with the SCF guidelines. The Netherlands notes that it has accepted, on a case-by-case basis, toxicity data sets deviating from the EFSA requirements in cases where the data was considered adequate to demonstrate the safe use of the substance.
MSs may use other MSs’ or the CoE’s restrictions on substances to set up their own lists of authorised substances. This implies that they recognise the risk assessment and management of that MS or of the CoE. It should be noted that proper documentation on the specific protocols on risk assessment by national bodies could not be retrieved from publicly available sources, nor were they provided in the different rounds of contacts with MSs. A summary of the information collected regarding who does what in the field of risk assessment in the EU is more specifically tabulated in the article Summary of information for risk assessment.
As regards industry, Plastics Europe has produced guidance for the risk assessment of non-listed substances (NLS) and non-intentionally added substances (NIAS) (Plastics Europe — Risk assessment of non-listed substances (NLS) and non-intentionally added substances (NIAS) under Article 19 of Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food). The document considers the exposure and toxicological assessment of a substance and its risk characterisation. With this guidance document, PlasticsEurope intends to explain how the plastics (plastic intermediate material) producers interpret and respond to their risk assessment obligations for NLSs and NIASs under Article 3 of the framework regulation (Regulation (EC) No 1935/2004) and Article 19 of the regulation on plastic materials and articles intended to come into contact with food (Regulation (EU) No 10/2011), based on internationally recognised tools and the scientific knowledge available to them at the time of writing. This is a living document that will be updated as and when needed.
CEPE published in 2009 the ‘Code of practice for coated articles where the food contact layer is a coating — working document’ (CEPE — Code of practice for coated articles where the food contact layer is a coating — working document, 2009.) that in Annex VI describes very briefly the risk assessment that should be performed by industry for migrants from coated articles in contact with foodstuffs. It divides the migrants into three classes, based on their use or nature, and provides some very basic directions to comply with Article 3 of the framework regulation.

